Rank the Following Bonds From Most Polar to Least Polar
Use numbers from 1. Given the electronegativity of each of the following atoms rank the bonds from most polar number 1 to least polar number 5.
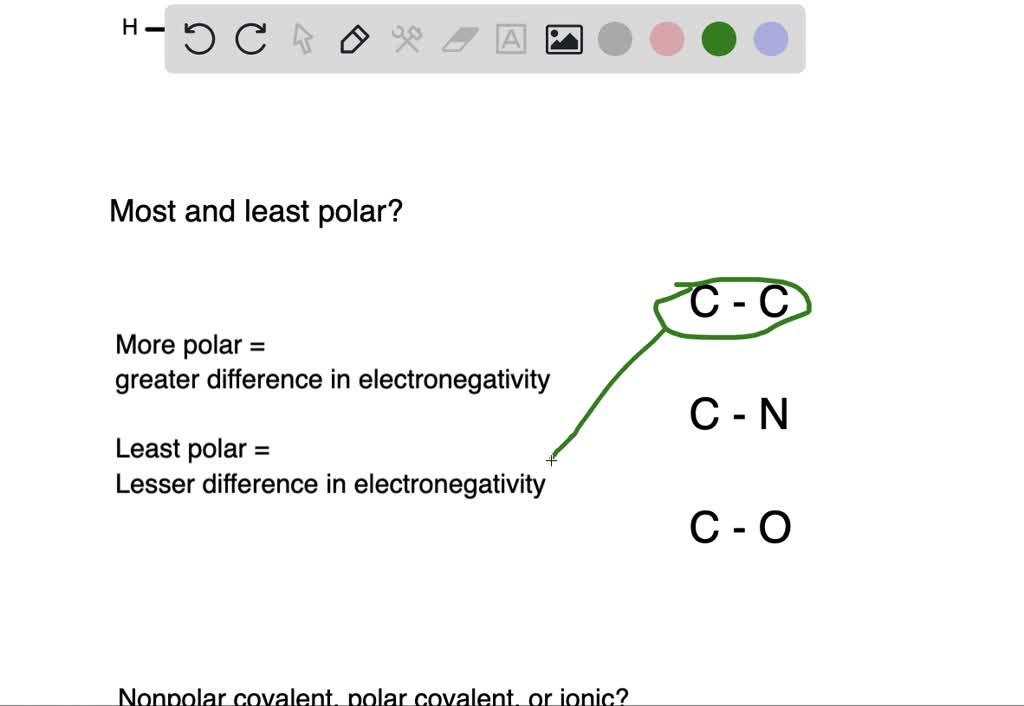
Rank the following bonds from most polar to least polar O-H C-H C-N C-F C-C Which of the following compounds are polar.
. Rank the following bonds from least polar to most polar. View the full answer. Chemistry questions and answers.
Assign formal charges to the atoms in the Lewis structure below. O 8 N 7 C. Which of the following bonds would be the most polar without being.
0 35 N 30 C 25 H 21. What is a Polar. Answer 1 of 28.
To find which one of the bond is least polar you need to find the electronegativity difference between the atoms that are involved in bondingthe more is the. Rank the following bonds from least polar to most polar. A water molecule is held together by two single polar covalent bonds.
Rank the following types of bonds by. This is the best answer based on feedback and ratings. Which of the following bonds would be the least polar yet still be considered polar covalent.
Polarity in Covalent bonds is due to the high difference in E. Rank the following bonds from most polar to least Sorting C-Cl C-F C-Br. 100 6 ratings take the electronegativity difference of both atoms in all.
The polarity of a bond depends on electronegativity difference between the two atoms sharing the bonding electrons. Elect to negativity. Mg-O C-O O-O Si-O N-O.
The polarity of the bonds can be determined on the basis o. View the full answer. Aus in polar bonds elect more electronegative atom pull election density towards itsey This polarity depends on electronegativity difference between two atoms.
The following are the ranking of solution from the least polar to most polar. First week only 499. View the full answer.
Most polar C-F Second most polar C-O Least polar C-N. Click here to get an answer to your question Rank the bonds from most polar to least polar. Start your trial now.
Three of the most. Rank the following bonds from least polar to most polar. Circle SO_2 CO_2 CCL_4 CH_2CL_2 HCN H_2O.
Increasing the electronegativity difference lead. Order the atoms by the number of covalent bonds they could form from least to most. Chemical Bonds and the Structure of Molecules Lewis dot structures of molecules show how electrons are shared in a molecule-A pair of shared electrons between two atoms is a bonding.
We want to rank these bonds by their polarity to one is the least polar. True or False. Solution for Rank the following bonds in the order of increasing polarity least polar to most polar a least polar C-C.
Chemistry questions and answers. Use numbers from 1 least to 4 most C-F. Looking at ionic her look surveillance nonproductive vince.
Rank the following bonds from most polar to least polar. A C-O C-F C-N b C-CI C-I C-Br c H-O H-N H-C d C-H C KittyGirl8265.

Solved 3 Rank The Following Bonds From Least Polar To Most Polar C C O H C O C N

Solved Rank The Following Bonds From Highest Polarity To The Lowest 1 Most Polar 4 Least Polar N Ci Select C Si Select O P Select A C Select
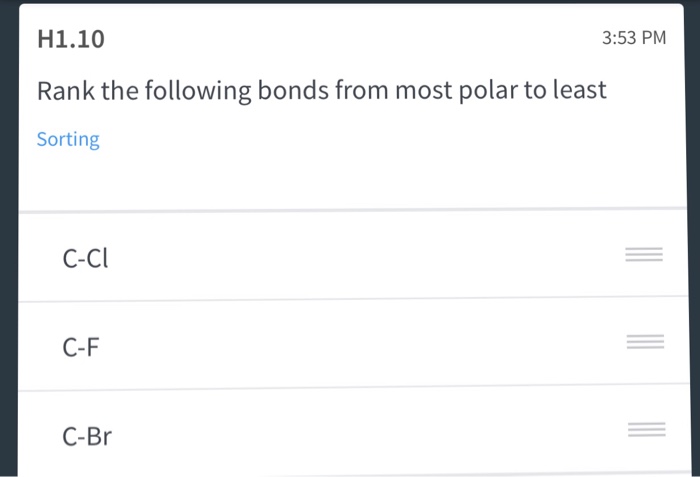
Solved Rank The Following Bonds From Most Polar To Least Chegg Com
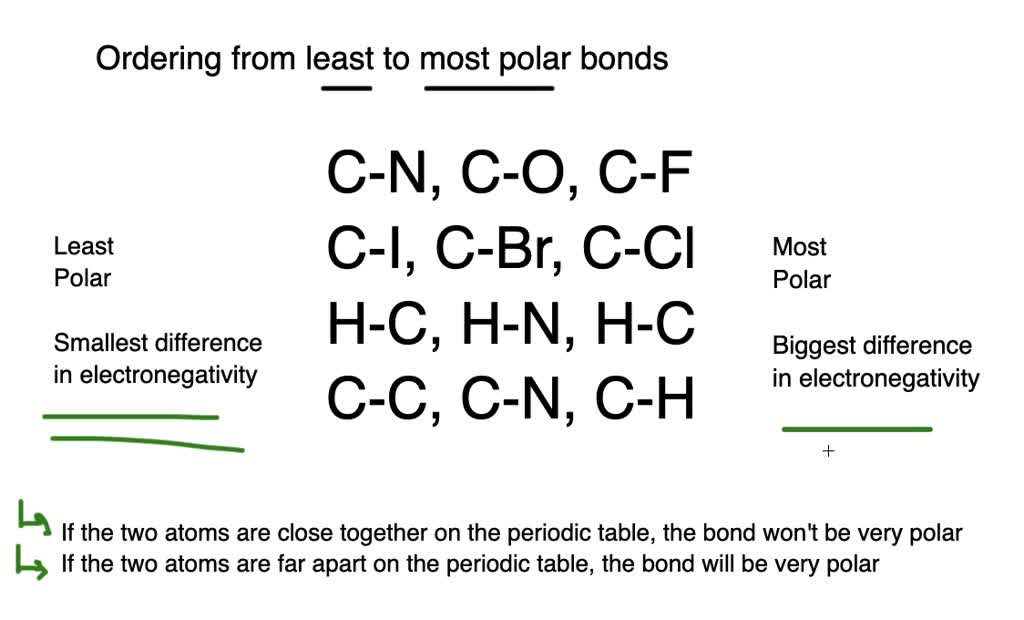
Solved Rank The Bonds From Most Polar To Least Polar A Quad Mathrm C Mathrm O Mathrm C Mathrm F Mathrm C Mathrm N B Quad Mathrm C Mathrm Cl Mathrm C Mathrm I Mathrm C Mathrm Br C Quad Mathrm H Mathrm O Mathrm H

Comments
Post a Comment